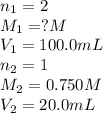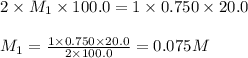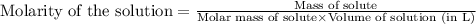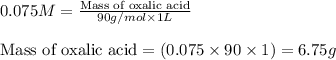Chemistry, 27.02.2020 05:47 utjfkdndidndldn62121
A sample of oxalic acid (a diprotic acid of the formula H2C2O4) is dissolved in enough water to make 1.00 L of solution. A 100.0 mL sample of this solution is titrated with a solution of sodium hydroxide of concentration 0.750 M and requires 20.0 mL of sodium hydroxide to reach the end point. Calculate the mass of the original oxalic acid sample.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

You know the right answer?
A sample of oxalic acid (a diprotic acid of the formula H2C2O4) is dissolved in enough water to make...
Questions

Mathematics, 04.12.2020 21:30


Mathematics, 04.12.2020 21:30

Mathematics, 04.12.2020 21:30


Mathematics, 04.12.2020 21:30

Chemistry, 04.12.2020 21:30



Mathematics, 04.12.2020 21:30



Mathematics, 04.12.2020 21:30



Mathematics, 04.12.2020 21:30

Spanish, 04.12.2020 21:30

Social Studies, 04.12.2020 21:30


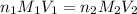
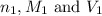 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 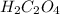
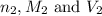 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.