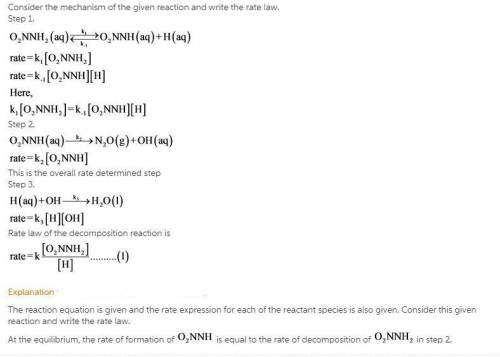
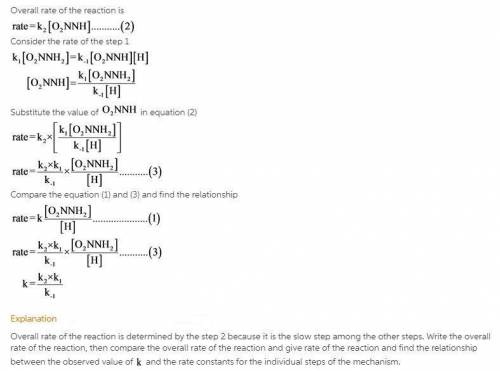
The decomposition of nitramide, O 2 NNH 2 , in water has the chemical equation and rate law O 2 NNH 2 ( aq ) ⟶ N 2 O ( g ) + H 2 O ( l ) rate = k [ O 2 NNH 2 ] [ H + ] A proposed mechanism for this reaction is O 2 NNH 2 ( aq ) k 1 ⇌ k − 1 O 2 NNH − ( aq ) + H + ( aq ) ( fast equilibrium ) O 2 NNH − ( aq ) k 2 −→ N 2 O ( g ) + OH − ( aq ) ( slow ) H + ( aq ) + OH − ( aq ) k 3 −→ H 2 O ( l ) ( fast ) What is the relationship between the observed value of k and the rate constants for the individual steps of the mechanism?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Many reactions take place in aqueous solution. when potential reactants are mixed, a reaction will occur if there is some driving force that favors the formation of products. it is often convenient to categorize reactions in terms of these driving forces: precipitate formation, in which an insoluble solid is formed, weak electrolyte formation, as in a neutralization reaction involving water, or transfer of electrons, as in a redox reaction. these reactions can be represented by full molecular equations, which contain all species in the reaction mixture, or by net ionic equations, which show only the species that actually undergo a change. the latter does not contain the spectator ions, which do not undergo a net change or do not take part in the reaction. part a when the following two solutions are mixed: k2co3(aq)+fe(no3)3(aq) the mixture contains the ions listed below. sort these species into spectator ions and ions that react. drag the appropriate items to their respective bins. view available hint(s) spectator ions ions that react part b what is the correct net ionic equation, including all coefficients, charges, and phases, for the following set of reactants? assume that the contribution of protons from h2so4 is near 100 %.ba(oh)2(aq)+h2so4(aq)→ express your answer as a chemical equation. view available hint(s) nothing provide feedback
Answers: 3

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3

Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1
You know the right answer?
The decomposition of nitramide, O 2 NNH 2 , in water has the chemical equation and rate law O 2 NNH...
Questions




History, 17.04.2020 01:13

Mathematics, 17.04.2020 01:13


History, 17.04.2020 01:13

Mathematics, 17.04.2020 01:13

Physics, 17.04.2020 01:13




Computers and Technology, 17.04.2020 01:13

Mathematics, 17.04.2020 01:13





English, 17.04.2020 01:13