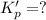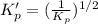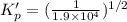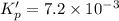Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1


Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
For the following reaction, Kp = 1.9 ✕ 104 at 1722 K. H2(g) + Br2(g) equilibrium reaction arrow 2 HB...
Questions





History, 20.07.2019 19:30

History, 20.07.2019 19:30


Spanish, 20.07.2019 19:30

History, 20.07.2019 19:30



Physics, 20.07.2019 19:30

Physics, 20.07.2019 19:30

Physics, 20.07.2019 19:30

Biology, 20.07.2019 19:30

History, 20.07.2019 19:30

History, 20.07.2019 19:30

Social Studies, 20.07.2019 19:30

Chemistry, 20.07.2019 19:30

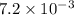
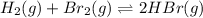 ;
; 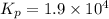
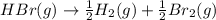 ;
;