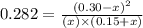Chemistry, 27.02.2020 02:01 FreyaLouise
He following reaction becomes possible: H2gI2g 2HIg The equilibrium constant K for this reaction is 0.282 at the temperature of the flask. Calculate the equilibrium molarity of H2. Round your answer to two decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1


Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1

Chemistry, 23.06.2019 10:00
Abike ride event is 30 miles. a first aid tent is put at the 3/4 mark of the course. how many miles from the starting point is the first aid tent?
Answers: 1
You know the right answer?
He following reaction becomes possible: H2gI2g 2HIg The equilibrium constant K for this reaction is...
Questions







Biology, 19.05.2020 02:20

History, 19.05.2020 02:20


Mathematics, 19.05.2020 02:20

Mathematics, 19.05.2020 02:20


Biology, 19.05.2020 02:20

Advanced Placement (AP), 19.05.2020 02:20


Advanced Placement (AP), 19.05.2020 02:20

Mathematics, 19.05.2020 02:20

English, 19.05.2020 02:20

Mathematics, 19.05.2020 02:20

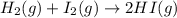
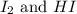
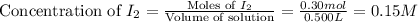
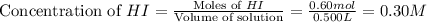
![K=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0526/1028/8a740.png)