Chemistry, 27.02.2020 00:31 dakotakeating4513
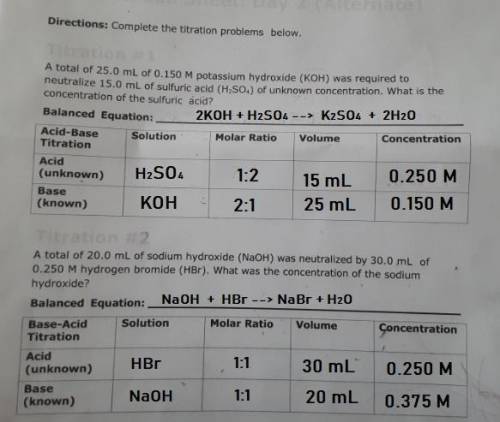
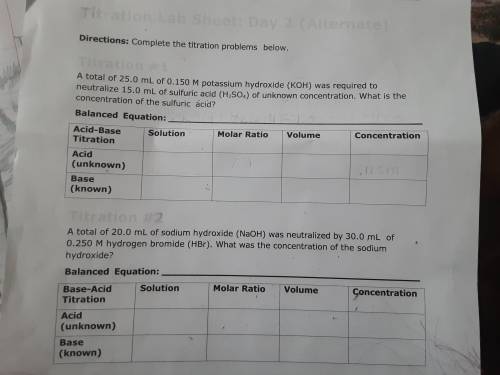
PLEASE HELP! A total of 25.0 mL of 0.150 M potassium hydroxide (KOH) was required to neutralize 15.0 mL of sulfuric acid (H2SO4) of unknown concentration. What is the concentration of the sulfuric acid?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
PLEASE HELP! A total of 25.0 mL of 0.150 M potassium hydroxide (KOH) was required to neutralize 15.0...
Questions

Mathematics, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40

Biology, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40

Biology, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40

Mathematics, 12.05.2021 23:40





Chemistry, 12.05.2021 23:40



English, 12.05.2021 23:40


English, 12.05.2021 23:40