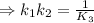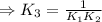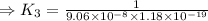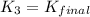Chemistry, 27.02.2020 00:10 barnhill6534
H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.06×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.18×10−19, what is the equilibrium constant Kfinal for the following reaction? S2−(aq)+2H+(aq)⇌H2S(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 13:30
Elaborate on the reason(s) that matter is said to move even as in a solid state. select one: a. the particles are bound through intermolecular forces but are able to move past each other with relative freedom. b. the particles have sufficient energy to become an ionized gas and are in the most common state of matter in the universe. c. the particles are not able to move out of their positions relative to one another, but do have small vibrational movements. d. the particles are not bound to one another, move quickly, have a low density, and are able to spread apart from one another if unconstrained.
Answers: 1
You know the right answer?
H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.06×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.18×10−19, what is the equ...
Questions



Advanced Placement (AP), 07.06.2021 19:20

Mathematics, 07.06.2021 19:20

Mathematics, 07.06.2021 19:20


History, 07.06.2021 19:20



English, 07.06.2021 19:20

Mathematics, 07.06.2021 19:20



Mathematics, 07.06.2021 19:20

Social Studies, 07.06.2021 19:20

Physics, 07.06.2021 19:20




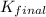 is 9.35× 10²⁵
is 9.35× 10²⁵![k=\frac{[C]^z}{[A]^x[B]^y}](/tpl/images/0525/8601/0a5aa.png)
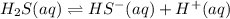
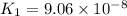
![K_1=\frac{[HS^-][H^+]}{[H_2S]}](/tpl/images/0525/8601/e1e1d.png)
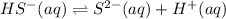
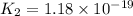
![K_2=\frac{[S^{2-}][H^+]}{[HS^-]}](/tpl/images/0525/8601/661de.png)
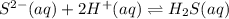
![K_3=\frac{[H_2S]}{[S^{2-}][H^+]^2}](/tpl/images/0525/8601/58537.png)
![k_1k_2=\frac{[HS^-][H^+]}{[H_2S]}\frac{[S^{2-}][H^+]}{[HS^-]}](/tpl/images/0525/8601/53894.png)
![\Rightarrow k_1k_2=\frac{[S^{2-}][H^+]^2}{[H_2S]}](/tpl/images/0525/8601/762f9.png)