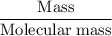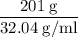Chemistry, 26.02.2020 23:21 annalaurie7
Methanol (CH3OH) burns in air according to the equation 2CH3OH + 3O2 → 2CO2 + 4H2O If 201 g of methanol are used up in a combustion process, what is the mass of H2O produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3


Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2
You know the right answer?
Methanol (CH3OH) burns in air according to the equation 2CH3OH + 3O2 → 2CO2 + 4H2O If 201 g of metha...
Questions

History, 05.05.2020 14:30

Mathematics, 05.05.2020 14:30



Engineering, 05.05.2020 14:30

Biology, 05.05.2020 14:30

Mathematics, 05.05.2020 14:30

Biology, 05.05.2020 14:30











Mathematics, 05.05.2020 14:30

Mathematics, 05.05.2020 14:30