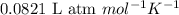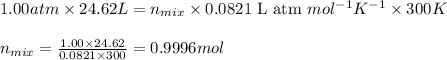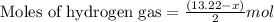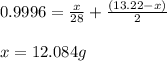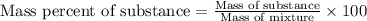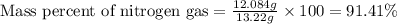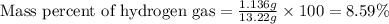Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Mixture of N 2 And H2 Gases weighs 13.22 g and occupies a volume of 24.62 L at 300 K and 1.00 atm. C...
Questions

Arts, 12.01.2021 23:30




Law, 12.01.2021 23:30

Mathematics, 12.01.2021 23:30

English, 12.01.2021 23:30

Mathematics, 12.01.2021 23:30

Mathematics, 12.01.2021 23:30


Biology, 12.01.2021 23:30


Mathematics, 12.01.2021 23:30




French, 12.01.2021 23:30


English, 12.01.2021 23:30