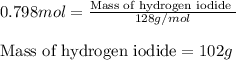Chemistry, 26.02.2020 22:32 crzyemo865
For the chemical reaction below, determine the amount of HI produced when 3.32E+0 g of hydrogen is reacted with 5.064E+1 g of iodine to produce hydrogen iodide (HI). H(g) + I(g) → 2HI(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1


Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
For the chemical reaction below, determine the amount of HI produced when 3.32E+0 g of hydrogen is r...
Questions

Geography, 02.11.2019 20:31

Health, 02.11.2019 20:31

Biology, 02.11.2019 20:31

Biology, 02.11.2019 20:31

Mathematics, 02.11.2019 20:31




History, 02.11.2019 20:31

History, 02.11.2019 20:31

Mathematics, 02.11.2019 20:31

History, 02.11.2019 20:31

Social Studies, 02.11.2019 20:31





Social Studies, 02.11.2019 20:31

Chemistry, 02.11.2019 20:31

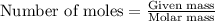 .....(1)
.....(1)
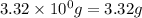
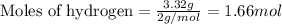
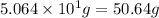
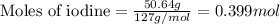
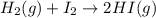
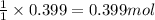 of hydrogen
of hydrogen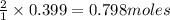 of hydrogen iodide
of hydrogen iodide