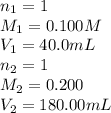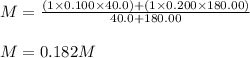Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1
You know the right answer?
A solution was prepared by mixing 40.00 mL of 0.100 M HNO3 and 180.00 mL of 0.200 M HNO3 . Calculate...
Questions

Health, 26.03.2021 01:00



Mathematics, 26.03.2021 01:00



Mathematics, 26.03.2021 01:00

History, 26.03.2021 01:00

Chemistry, 26.03.2021 01:00


Engineering, 26.03.2021 01:00



English, 26.03.2021 01:00


Arts, 26.03.2021 01:00




Geography, 26.03.2021 01:00

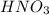 in the final solution is 0.182 M
in the final solution is 0.182 M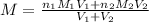
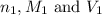 are the n-factor, molarity and volume of the
are the n-factor, molarity and volume of the 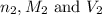 are the n-factor, molarity and volume of the
are the n-factor, molarity and volume of the