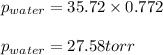Chemistry, 26.02.2020 20:52 cocomorillo35181
The ambient temperature is 85.0°F and the humidity of the surrounding air is reported to be 68.0%. Using the Clausius-Clapeyron equation and the boiling point of water as 100.0°C at 760 torr, calculate the vapor pressure (in torr) of water in the air. Use 40.7 kJ/mol as the ∆Hvap of water.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3

Chemistry, 23.06.2019 10:00
How many grams of cupric sulfate pentahydrate are needed to prepare 50.00 ml of 0.0800m cuso4× 5h2o?
Answers: 3
You know the right answer?
The ambient temperature is 85.0°F and the humidity of the surrounding air is reported to be 68.0%. U...
Questions


Mathematics, 05.10.2020 19:01

History, 05.10.2020 19:01


Business, 05.10.2020 19:01

History, 05.10.2020 19:01



History, 05.10.2020 19:01


English, 05.10.2020 19:01


Mathematics, 05.10.2020 19:01

Mathematics, 05.10.2020 19:01






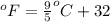
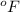 = temperature in Fahrenheit
= temperature in Fahrenheit 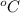 = temperature in centigrade
= temperature in centigrade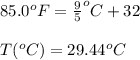
![\ln(\frac{P_2}{P_1})=\frac{\Delta H}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0525/3984/5c76e.png)
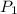 = initial pressure which is the pressure at normal boiling point = 760 torr
= initial pressure which is the pressure at normal boiling point = 760 torr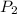 = final pressure = ?
= final pressure = ?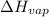 = Enthalpy of vaporization = 40.7 kJ/mol = 40700 J/mol (Conversion factor: 1 kJ = 1000 J)
= Enthalpy of vaporization = 40.7 kJ/mol = 40700 J/mol (Conversion factor: 1 kJ = 1000 J)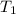 = initial temperature =
= initial temperature = ![100^oC=[100+273]K=373K](/tpl/images/0525/3984/44e24.png)
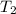 = final temperature =
= final temperature = ![29.44^oC=[29.44+273]=302.44K](/tpl/images/0525/3984/ddd83.png)
![\ln(\frac{P_2}{760})=\frac{40700J/mol}{8.314J/mol.K}[\frac{1}{373}-\frac{1}{302.44}]\\\\P_2=35.72torr](/tpl/images/0525/3984/e0926.png)
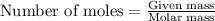
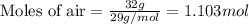
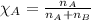

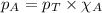
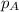 = vapor pressure of water = ?
= vapor pressure of water = ?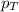 = total pressure = 35.72 torr
= total pressure = 35.72 torr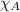 = mole fraction of water = 0.774
= mole fraction of water = 0.774