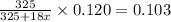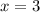Chemistry, 26.02.2020 18:29 unknown337
4. A 120.0 mg sample of a lead acetate hydrate, Pb(C2H3O2)2 × xH2O was heated to drive off the waters of hydration. The cooled residue had a mass of 103.0 mg. Calculate the value of x in the chemical formula. Show your work with Equation Editor.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1
You know the right answer?
4. A 120.0 mg sample of a lead acetate hydrate, Pb(C2H3O2)2 × xH2O was heated to drive off the wate...
Questions

Mathematics, 15.05.2021 01:20



Mathematics, 15.05.2021 01:20






Mathematics, 15.05.2021 01:20



Mathematics, 15.05.2021 01:20

Mathematics, 15.05.2021 01:20

Mathematics, 15.05.2021 01:20

Chemistry, 15.05.2021 01:20


Spanish, 15.05.2021 01:20

Mathematics, 15.05.2021 01:20

English, 15.05.2021 01:20

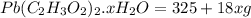
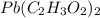 = 325 g
= 325 g
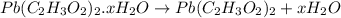
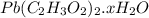 on heating gives = 325 g of
on heating gives = 325 g of 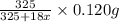 of
of