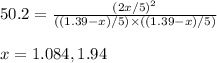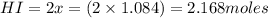Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
Calculate the number of moles of HI that are at equilibrium with 1.39 mol of H2 and 1.39 mol of I2 i...
Questions





German, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50



Mathematics, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50




History, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50



Mathematics, 18.12.2020 02:50

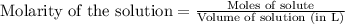
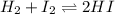
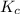 for above equation follows:
for above equation follows:![K_c=\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/0525/0428/8acbe.png)
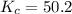
![[HI]_{eq}=\frac{2x}{5.00}](/tpl/images/0525/0428/ab69d.png)
![[H_2]_{eq}=\frac{(1.39-x)}{5.00}](/tpl/images/0525/0428/598c6.png)
![[I_2]_{eq}=\frac{(1.39-x)}{5.00}](/tpl/images/0525/0428/0f5f3.png)