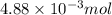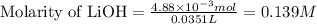Chemistry, 26.02.2020 17:58 gamerdoesart
In a precipitation reaction between FeCl2(aq) and LiOH(aq), 11.6 mL of 0.210 M FeCl2(aq) completly reacted with 35.1 mL of LiOH(aq). What was the molarity of LiOH(aq)? FeCl2(aq) + 2 LiOH(aq) → Fe(OH)2(s) + 2 LiCl(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

You know the right answer?
In a precipitation reaction between FeCl2(aq) and LiOH(aq), 11.6 mL of 0.210 M FeCl2(aq) completly r...
Questions


Mathematics, 26.07.2019 23:00



Social Studies, 26.07.2019 23:00


English, 26.07.2019 23:00

Mathematics, 26.07.2019 23:00


Social Studies, 26.07.2019 23:00

English, 26.07.2019 23:00


Business, 26.07.2019 23:00

Mathematics, 26.07.2019 23:00



Mathematics, 26.07.2019 23:00

English, 26.07.2019 23:00

Mathematics, 26.07.2019 23:00

Biology, 26.07.2019 23:00

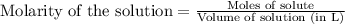 .....(1)
.....(1)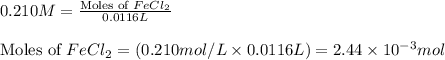
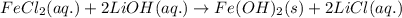
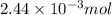 moles of iron (II) chloride will react with =
moles of iron (II) chloride will react with = 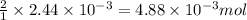 of LiOH
of LiOH