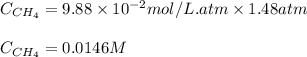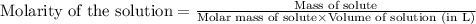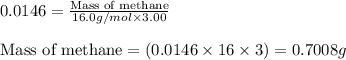Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
Methane (CH4, molar mass = 16.0 g/mol) has a Henry's Law constant (kH) of 9.88 × 10–2 mol/(L·atm) wh...
Questions

History, 16.07.2019 14:00

English, 16.07.2019 14:00




Mathematics, 16.07.2019 14:00


Mathematics, 16.07.2019 14:00

English, 16.07.2019 14:00


Mathematics, 16.07.2019 14:00

History, 16.07.2019 14:00

Mathematics, 16.07.2019 14:00

Mathematics, 16.07.2019 14:00

Mathematics, 16.07.2019 14:00

English, 16.07.2019 14:00


Mathematics, 16.07.2019 14:00

Mathematics, 16.07.2019 14:00

Biology, 16.07.2019 14:00

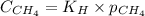
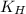 = Henry's constant =
= Henry's constant = 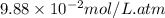
 = molar solubility of methane gas = ?
= molar solubility of methane gas = ?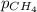 = partial pressure of methane gas = 1.48 atm
= partial pressure of methane gas = 1.48 atm