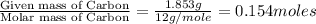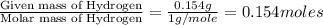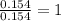Chemistry, 26.02.2020 16:40 kayla942783
A 2.007 g sample of a hydrocarbon is combusted to give 1.389 g of H 2O and 6.785 g of CO 2. What is the empirical formula of the compound? a. CH2 b. C2Hw c. C3H4 d. CH e. C5H12

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
A 2.007 g sample of a hydrocarbon is combusted to give 1.389 g of H 2O and 6.785 g of CO 2. What is...
Questions



Mathematics, 13.05.2021 06:10

Biology, 13.05.2021 06:10

Mathematics, 13.05.2021 06:10

Mathematics, 13.05.2021 06:20

Mathematics, 13.05.2021 06:20


Mathematics, 13.05.2021 06:20


Mathematics, 13.05.2021 06:20

History, 13.05.2021 06:20

Mathematics, 13.05.2021 06:20


English, 13.05.2021 06:20

Mathematics, 13.05.2021 06:20


Mathematics, 13.05.2021 06:20

Mathematics, 13.05.2021 06:20

Geography, 13.05.2021 06:20

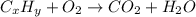
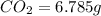
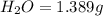
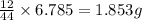 of carbon will be contained.
of carbon will be contained.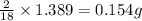 of hydrogen will be contained.
of hydrogen will be contained.