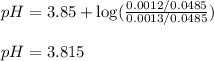Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2


Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
A student performs a titration of 25.0 mL of 0.100 M lactic acid (HC3H5O3), using 0.050 M sodium hyd...
Questions

History, 16.09.2019 03:30

Mathematics, 16.09.2019 03:30

Biology, 16.09.2019 03:30


Biology, 16.09.2019 03:30

Biology, 16.09.2019 03:30

History, 16.09.2019 03:30



Mathematics, 16.09.2019 03:30

Mathematics, 16.09.2019 03:30


History, 16.09.2019 03:30

Mathematics, 16.09.2019 03:30

Chemistry, 16.09.2019 03:30

Mathematics, 16.09.2019 03:30




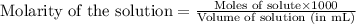
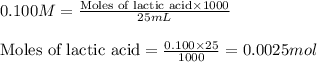
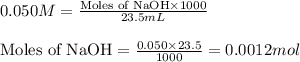
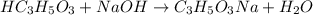
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0524/8883/e4eea.png)
![pH=pK_a+\log(\frac{[C_3H_5O_3Na]}{[HC_3H_5O_3]})](/tpl/images/0524/8883/28764.png)
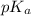 = negative logarithm of acid dissociation constant of lactic acid = 3.85
= negative logarithm of acid dissociation constant of lactic acid = 3.85![[HC_3H_5O_3]=\frac{0.0013}{0.0485}](/tpl/images/0524/8883/28573.png)
![[C_3H_5O_3Na]=\frac{0.0012}{0.0485}](/tpl/images/0524/8883/f1a5a.png)