
Chemistry, 26.02.2020 16:25 michaela134
A mixture of 0.439 M H 2 , 0.317 M I 2 , and 0.877 M HI is enclosed in a vessel and heated to 430 °C. H 2 ( g ) + I 2 ( g ) − ⇀ ↽ − 2 HI ( g ) K c = 54.3 at 430 ∘ C Calculate the equilibrium concentrations of each gas at 430 ∘ C .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1


Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
A mixture of 0.439 M H 2 , 0.317 M I 2 , and 0.877 M HI is enclosed in a vessel and heated to 430 °C...
Questions



English, 26.03.2021 03:50


Mathematics, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

Computers and Technology, 26.03.2021 03:50

Law, 26.03.2021 03:50


History, 26.03.2021 03:50


Mathematics, 26.03.2021 03:50

Advanced Placement (AP), 26.03.2021 03:50


Mathematics, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

Biology, 26.03.2021 03:50

Mathematics, 26.03.2021 03:50

English, 26.03.2021 03:50

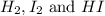 at equilibrium is, 0.244 M, 0.122 M and 1.267 M respectively.
at equilibrium is, 0.244 M, 0.122 M and 1.267 M respectively.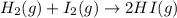
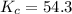
![K=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0524/8903/8a740.png)
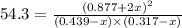
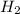 at equilibrium = (0.439-x) = (0.439-0.195) = 0.244 M
at equilibrium = (0.439-x) = (0.439-0.195) = 0.244 M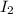 at equilibrium = (0.317-x) = (0.317-0.195) = 0.122 M
at equilibrium = (0.317-x) = (0.317-0.195) = 0.122 M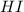 at equilibrium = (0.877+2x) = (0.877+2\times 0.195) = 1.267 M
at equilibrium = (0.877+2x) = (0.877+2\times 0.195) = 1.267 M



