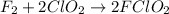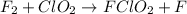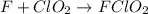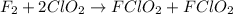Chemistry, 26.02.2020 04:19 kaylabay1997
A mechanism for the gas phase reaction of fluorine with chlorine dioxide that is consistent with the observed rate law is:
step 1 slow: F2 + ClO2 > FClO2 + F
step 2 fast: F > ClO2 + FClO2
What is the equation for the overall reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
A mechanism for the gas phase reaction of fluorine with chlorine dioxide that is consistent with the...
Questions



Social Studies, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00







Mathematics, 03.12.2020 01:00



Mathematics, 03.12.2020 01:00

Chemistry, 03.12.2020 01:00

History, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00


German, 03.12.2020 01:00