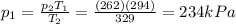On a summer day, you take a road trip through Death Valley, California, in an antique car. You start out at a temperature of 21°C, but the temperature in Death Valley will reach a peak of 56°C. The tires on your car hold 13.2 L of nitrogen gas at a starting pressure of 240 kPa. The tires will burst when the internal pressure (Pb) reaches 262 kPa. Answer the following questions and show your work.
• How many moles of nitrogen gas are in each tire?
• What will the tire pressure be at peak temperature in Death Valley?
• Will the tires burst in Death Valley? Explain.
• If you must let nitrogen gas out of the tire before you go, to what pressure must you reduce the tires before you start your trip? (Assume no significant change in tire volume.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3

You know the right answer?
On a summer day, you take a road trip through Death Valley, California, in an antique car. You start...
Questions




History, 18.07.2019 15:50

Mathematics, 18.07.2019 15:50




Spanish, 18.07.2019 15:50


Chemistry, 18.07.2019 15:50

Health, 18.07.2019 15:50

Mathematics, 18.07.2019 15:50




Mathematics, 18.07.2019 15:50



Biology, 18.07.2019 15:50

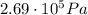
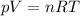
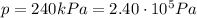
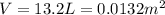
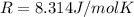
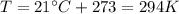
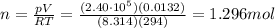
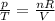
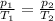
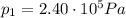 is the initial pressure
is the initial pressure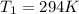 is the initial temperature
is the initial temperature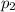 is the final pressure in Death Valley
is the final pressure in Death Valley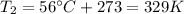 is the temperature in Death Valley
is the temperature in Death Valley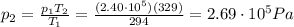
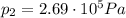
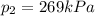
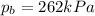
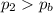
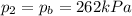
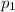 , the initial pressure at which the tires should be in order not to burst when the car arrives in the Death Valley.
, the initial pressure at which the tires should be in order not to burst when the car arrives in the Death Valley.