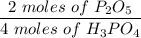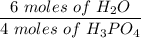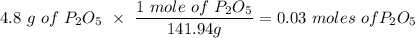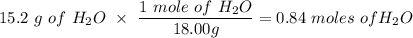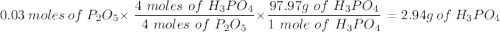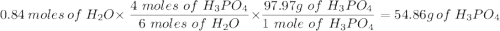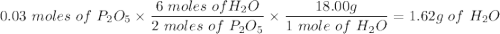Chemistry, 26.02.2020 02:54 preciousharrington13
Given this equation: 2P2O5 + 6H2O ---> 4H3PO4.
If you begin with 4.8 grams of P205 and 15.2 grams H2O, what will be your limiting reactant? How many grams of H3PO4 are produced?
Whenever I multiply P2O5 I always get 3.3 grams rather than 6.6 grams. However, when I multiply H2O I get the correct answer.
How many grams of the excess reagent remain unreacted?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
Given this equation: 2P2O5 + 6H2O ---> 4H3PO4.
If you begin with 4.8 grams of P205 and...
If you begin with 4.8 grams of P205 and...
Questions








Biology, 22.10.2019 04:50



Mathematics, 22.10.2019 04:50

Biology, 22.10.2019 04:50

Biology, 22.10.2019 04:50





Law, 22.10.2019 04:50

Geography, 22.10.2019 04:50

Business, 22.10.2019 04:50