
Chemistry, 26.02.2020 01:46 231cornelius
Consider the reaction 2 NO + O2 → 2 NO2
Suppose that at a particular moment during the reaction nitric oxide (NO) is reacting at the rate of 0.050 M/s.
(a) At what rate is NO2 being formed?
(b) At what rate is molecular oxygen reacting?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3


Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1
You know the right answer?
Consider the reaction 2 NO + O2 → 2 NO2
Suppose that at a particular moment during the reactio...
Suppose that at a particular moment during the reactio...
Questions



History, 29.01.2020 08:42

Mathematics, 29.01.2020 08:42

Mathematics, 29.01.2020 08:42


Biology, 29.01.2020 08:43


History, 29.01.2020 08:43

English, 29.01.2020 08:43

Biology, 29.01.2020 08:43

World Languages, 29.01.2020 08:43

English, 29.01.2020 08:43

Health, 29.01.2020 08:43

Business, 29.01.2020 08:43

Mathematics, 29.01.2020 08:43

History, 29.01.2020 08:43


History, 29.01.2020 08:43

Social Studies, 29.01.2020 08:43

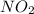 is formed is 0.050 M/s
is formed is 0.050 M/s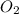 is consumed is 0.0250 M/s.
is consumed is 0.0250 M/s.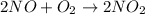
![-\frac{1}{2}\frac{d[NO]}{dt}=-\frac{d[O_2]}{dt}=\frac{1}{2}\frac{d[NO_2]}{dt}](/tpl/images/0524/2208/cb5a7.png)
![\frac{d[NO]}{dt}=-0.050\ M/s](/tpl/images/0524/2208/0265a.png) (Negative sign shows consumption)
(Negative sign shows consumption)![-\frac{1}{2}\frac{d[NO]}{dt}=\frac{1}{2}\frac{d[NO_2]}{dt}](/tpl/images/0524/2208/0085e.png)
![-\frac{d[NO]}{dt}=\frac{d[NO_2]}{dt}](/tpl/images/0524/2208/49698.png)
![-(-0.050\ M/s)=\frac{d[NO_2]}{dt}](/tpl/images/0524/2208/98254.png)
![\frac{d[NO_2]}{dt}=0.050\ M/s](/tpl/images/0524/2208/27395.png)
![-\frac{1}{2}\frac{d[NO]}{dt}=-\frac{d[O_2]}{dt}](/tpl/images/0524/2208/fa609.png)
![-\frac{1}{2}\times -0.050\ M/s=-\frac{d[O_2]}{dt}](/tpl/images/0524/2208/649b1.png)
![\frac{d[O_2]}{dt}=0.0250\ M/s](/tpl/images/0524/2208/bc002.png)


