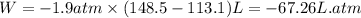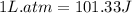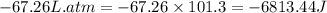Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
The combustion of 0.25 moles of octane gas (C8H18) to CO2 gas and H2O gas (vapor) against a constant...
Questions



History, 29.08.2019 10:20

Physics, 29.08.2019 10:20

History, 29.08.2019 10:20

Physics, 29.08.2019 10:20








Mathematics, 29.08.2019 10:20



History, 29.08.2019 10:20

English, 29.08.2019 10:20


Mathematics, 29.08.2019 10:20

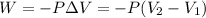
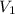 = initial volume = 113.1 L
= initial volume = 113.1 L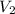 = final volume = 148.5 L
= final volume = 148.5 L