Consider the following elementary reaction:
N2O—> N2 + O
suppose we let K1 stand fo...

Consider the following elementary reaction:
N2O—> N2 + O
suppose we let K1 stand for the rate constant of this reaction, and k-1 stand for the rate constant of the reverse reaction. write an expression that gives the equilibrium concentration of N2O in terms of k1, k-1 and the equilibrium concentrations of N2 and O

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
Questions

Social Studies, 29.01.2020 04:05

Mathematics, 29.01.2020 04:05


Physics, 29.01.2020 04:05




Mathematics, 29.01.2020 04:05


Mathematics, 29.01.2020 04:05


English, 29.01.2020 04:05


History, 29.01.2020 04:05


Mathematics, 29.01.2020 04:05

Mathematics, 29.01.2020 04:05



History, 29.01.2020 04:05

![[N_2O]=\frac{K^{-1}}{K_1}\times [N_2][O_2]](/tpl/images/0524/0986/7a4ba.png)
![R_f=K_1\times [N_2O]](/tpl/images/0524/0986/5d806.png)
![R_b=K^{-1}\times [N_2][O_2]](/tpl/images/0524/0986/327e3.png)
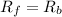
![K_1\times [N_2O]=K^{-1}\times [N_2][O_2]](/tpl/images/0524/0986/21d13.png)


