
PART A: Use the following glycolytic reaction to answer the question. If the concentration of DHAP is 0.125 M and the concentration of G3P is 0.06 M in a cell, what is the free energy change (ΔG)? Give your answer in 3 significant figures. (NOTE: Units are asked for in Part B.)
DHAP -> G3P (reversible)
Keq=5.4e-2
PART B: Units are important for all mathematical calculations. What are the units for free energy change in Part A?
PART C: Select ALL answers that correctly complete this sentence. Based on the calculated free energy change in Part A, the
a) forward reaction is favorable
b)forward reaction is unfavorable
c) reverse reaction is favorable
d) reverse reaction is unfavorable.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
PART A: Use the following glycolytic reaction to answer the question. If the concentration of DHAP i...
Questions

Biology, 16.11.2020 07:50


Biology, 16.11.2020 07:50

English, 16.11.2020 07:50

Mathematics, 16.11.2020 07:50




Mathematics, 16.11.2020 07:50




English, 16.11.2020 07:50



Mathematics, 16.11.2020 07:50

Mathematics, 16.11.2020 07:50


Mathematics, 16.11.2020 07:50

Mathematics, 16.11.2020 07:50

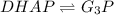
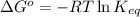
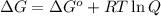
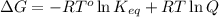
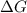 = Free energy change
= Free energy change 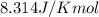
 = standard temperature =
= standard temperature = ![25^oC=[273+25]K=298K](/tpl/images/0523/9218/0e82f.png)
![37^oC=[273+37]K=310K](/tpl/images/0523/9218/c6b28.png)
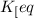 = equilibrium constant =
= equilibrium constant = 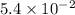
![\frac{[G_3P]}{[DHAP]}](/tpl/images/0523/9218/12d19.png)
![[G_3P]](/tpl/images/0523/9218/572f0.png) = 0.06 M
= 0.06 M![\Delta G=[-(8.314J/mol.K\times 298K\times \ln (5.4\times 10^{-2}))]+[(8.314J/mol.K\times 310K\times \ln (\frac{0.06}{0.125}))]\\\\\Delta G=-[-7231.46]+[-1891.7]=-5339.76J/mol](/tpl/images/0523/9218/63161.png)



