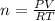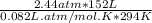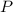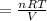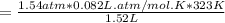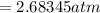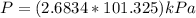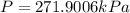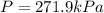On a summer day, you take a road trip through Moses Lake, WA, in a sports car. You start out at a temperature of 21°C in the morning, but the temperature in Moses Lake will reach a peak of 55°C. Each tire on your car holds 15.2 L of nitrogen gas at a starting pressure of 247 kPa. The tires will burst if the internal pressure exceeds 270 kPa.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1
You know the right answer?
On a summer day, you take a road trip through Moses Lake, WA, in a sports car. You start out at a te...
Questions

Business, 06.06.2020 07:57

Biology, 06.06.2020 07:57



Biology, 06.06.2020 07:57

English, 06.06.2020 07:57





Mathematics, 06.06.2020 07:57



Health, 06.06.2020 07:57

Mathematics, 06.06.2020 07:57

Biology, 06.06.2020 07:57

Mathematics, 06.06.2020 07:57

Chemistry, 06.06.2020 07:57

Mathematics, 06.06.2020 07:57

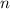
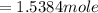
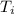 ) = 21°C = (21 +273)K
) = 21°C = (21 +273)K