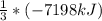Chemistry, 25.02.2020 22:49 alicciardone01
The enthalpy change for the explosion of ammonium nitrate with fuel oil is –7198 kJ for every 3 moles of NH4NO3. What is the enthalpy change for 1.0 mole of NH4NO3 in this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
The enthalpy change for the explosion of ammonium nitrate with fuel oil is –7198 kJ for every 3 mole...
Questions

History, 01.08.2019 09:30

Social Studies, 01.08.2019 09:30

Mathematics, 01.08.2019 09:30

Social Studies, 01.08.2019 09:30

Mathematics, 01.08.2019 09:30




History, 01.08.2019 09:30

History, 01.08.2019 09:30

Spanish, 01.08.2019 09:30

Mathematics, 01.08.2019 09:30


History, 01.08.2019 09:30

History, 01.08.2019 09:30

History, 01.08.2019 09:30




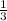 of the total enthaply of the 3 moles
of the total enthaply of the 3 moles