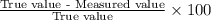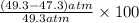Chemistry, 25.02.2020 21:59 sierram298
In Part B the given conditions were 1.00 mol of argon in a 0.500-L container at 27.0 ∘C . You identified that the ideal pressure (Pideal) was 49.3 atm , and the real pressure (Preal) was 47.3 atm under these conditions. The percent difference between the ideal and real gas is .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
In Part B the given conditions were 1.00 mol of argon in a 0.500-L container at 27.0 ∘C . You identi...
Questions

Mathematics, 12.07.2019 13:00


Mathematics, 12.07.2019 13:00



Mathematics, 12.07.2019 13:00


History, 12.07.2019 13:00





Business, 12.07.2019 13:00

Biology, 12.07.2019 13:00




Mathematics, 12.07.2019 13:00

Social Studies, 12.07.2019 13:00

Social Studies, 12.07.2019 13:00