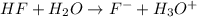Chemistry, 25.02.2020 20:18 21megoplin
Write the formula for the conjugate base of each of the following acids. Part A HCl Express your answer as a chemical formula. nothing Request Answer Part B H3PO4 Express your answer as a chemical formula. nothing Request Answer Part C HCN Express your answer as a chemical formula. nothing Request Answer Part D HF

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1


Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
Write the formula for the conjugate base of each of the following acids. Part A HCl Express your ans...
Questions

Computers and Technology, 24.05.2021 16:50


Social Studies, 24.05.2021 16:50

Computers and Technology, 24.05.2021 16:50


Mathematics, 24.05.2021 16:50


Biology, 24.05.2021 16:50

Mathematics, 24.05.2021 16:50




Computers and Technology, 24.05.2021 16:50


Business, 24.05.2021 16:50



Mathematics, 24.05.2021 16:50

Spanish, 24.05.2021 16:50

Mathematics, 24.05.2021 16:50

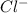 .
.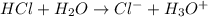
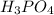 is
is 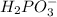 .
.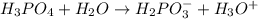
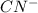 .
.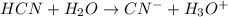
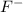 .
.