
The reaction below is carried out at a different temperature at which Kc=0.055. This time, however, the reaction mixture starts with only the product, [NO]=0.0100M, and no reactants. Find the equilibrium concentrations of N2, O2, and NO at equilibrium. N2(g)+O2(g)⇌2NO(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What are the concentrations of hydroxide and hydronium ions in a solution with a ph of 10.2?
Answers: 1

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

You know the right answer?
The reaction below is carried out at a different temperature at which Kc=0.055. This time, however,...
Questions

Mathematics, 04.08.2019 02:30

Social Studies, 04.08.2019 02:30

History, 04.08.2019 02:30

Biology, 04.08.2019 02:30

History, 04.08.2019 02:30


Social Studies, 04.08.2019 02:30



Social Studies, 04.08.2019 02:30

History, 04.08.2019 02:30



Biology, 04.08.2019 02:30


Social Studies, 04.08.2019 02:30

History, 04.08.2019 02:30



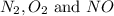 at equilibrium is, 0.0045 M, 0.0045 M and 0.001 M respectively.
at equilibrium is, 0.0045 M, 0.0045 M and 0.001 M respectively.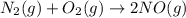
![K=\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0523/5256/ef70d.png)
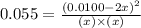
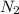 at equilibrium = x = 0.0045 M
at equilibrium = x = 0.0045 M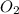 at equilibrium = x = 0.0045 M
at equilibrium = x = 0.0045 M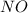 at equilibrium = (0.0100-2x) = (0.0100-2(0.0045)) = 0.001 M
at equilibrium = (0.0100-2x) = (0.0100-2(0.0045)) = 0.001 M

