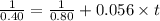Chemistry, 25.02.2020 19:44 tinajackson6534
In the reaction, A → Products, a plot of 1/[A] vs. time is linear and the slope is equal to 0.056 M−1 s−1. If the initial concentration of A is 0.80 M, how long will it take one-half of the initial amount of A to react?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1
You know the right answer?
In the reaction, A → Products, a plot of 1/[A] vs. time is linear and the slope is equal to 0.056 M−...
Questions

Arts, 17.12.2020 20:50

Mathematics, 17.12.2020 20:50

Mathematics, 17.12.2020 20:50

Mathematics, 17.12.2020 20:50


History, 17.12.2020 20:50

Health, 17.12.2020 20:50

History, 17.12.2020 20:50

Mathematics, 17.12.2020 20:50

Mathematics, 17.12.2020 20:50


Mathematics, 17.12.2020 20:50


Mathematics, 17.12.2020 20:50


Mathematics, 17.12.2020 20:50

Business, 17.12.2020 20:50

Geography, 17.12.2020 20:50

Mathematics, 17.12.2020 20:50

Mathematics, 17.12.2020 20:50

![\frac{1}{[A_t]} = \frac{1}{[A]_0}+kt](/tpl/images/0523/4978/f2ee3.png)
![[A_t]](/tpl/images/0523/4978/5262c.png) is the final concentration = Half of the initial concentration = 0.80 /2 M = 0.40 M
is the final concentration = Half of the initial concentration = 0.80 /2 M = 0.40 M![[A_0]](/tpl/images/0523/4978/9a686.png) is the initial concentration = 0.80 M
is the initial concentration = 0.80 M