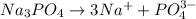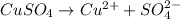Scoring: Your score will be based on the number of correct matches minus the number of incorrect matches. There is no penalty for missing matches.
A) Match the solutions to the descriptions of the freezing points.
Solutions:
1. One mole of the jonic compound Na3PO4 dissolved in 1000 g of H2O
2. One mole of the ionic compound CuSO4 dissolved in 1000 g H2O
3. One mole of the nonelectrolyte C6H12O6 dissolved in 1000 g H2O
Descriptions:
O lowest freezing point
O highest freezing point
O intermediate freezing point

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

You know the right answer?
Scoring: Your score will be based on the number of correct matches minus the number of incorrect mat...
Questions




Biology, 05.10.2020 15:01

English, 05.10.2020 15:01



Mathematics, 05.10.2020 15:01


History, 05.10.2020 15:01

History, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

English, 05.10.2020 15:01



Mathematics, 05.10.2020 15:01


Mathematics, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

English, 05.10.2020 15:01

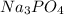 dissolved in 1000 g of
dissolved in 1000 g of 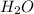 → lowest freezing point
→ lowest freezing point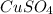 dissolved in 1000 g of
dissolved in 1000 g of 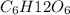 dissolved in 1000 g of
dissolved in 1000 g of 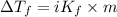
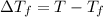
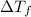 =depression in freezing point =
=depression in freezing point = 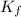 = freezing point constant
= freezing point constant 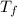 = freezing point of solution
= freezing point of solution