
Chemistry, 25.02.2020 06:22 Itsyourgirllulu
In the contact process, sulfuric acid is manufactured by first oxidizing SO2 to SO3, which is then reacted with water. The reaction of SO2 with O2 is
2SO2(g) 1 O2(g) m 2SO3(g)
A 2.000-L flask was filled with 0.0400 mol SO2 and 0.0200 mol O2. At equilibrium at 900 K, the flask contained 0.0296 mol SO3. How many moles of each substance were in the flask at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1


Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

You know the right answer?
In the contact process, sulfuric acid is manufactured by first oxidizing SO2 to SO3, which is then r...
Questions


Mathematics, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31


Mathematics, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31

Physics, 15.01.2020 06:31



Mathematics, 15.01.2020 06:31



Mathematics, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31

Mathematics, 15.01.2020 06:31



Mathematics, 15.01.2020 06:31

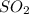 =0.0104
=0.0104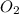 =0.0052
=0.0052

