
A compound containing chromium, Cr; chlorine, Cl; and oxygen, O, is analyzed and found to be 33.6% chromium, 45.8% chlorine, and 20.6% oxygen by mass. What is the empirical formula of the compound? The molar mass of chromium, Cr, is 51.996 gmol; the molar mass of chlorine, Cl, is 35.45 gmol; and the molar mass of oxygen, O, is 15.999 gmol.

Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
A compound containing chromium, Cr; chlorine, Cl; and oxygen, O, is analyzed and found to be 33.6% c...
Questions

Advanced Placement (AP), 17.12.2020 23:10

English, 17.12.2020 23:10




Mathematics, 17.12.2020 23:10


Mathematics, 17.12.2020 23:10


English, 17.12.2020 23:10

Chemistry, 17.12.2020 23:10

English, 17.12.2020 23:10


Mathematics, 17.12.2020 23:10

History, 17.12.2020 23:10



Business, 17.12.2020 23:10


Mathematics, 17.12.2020 23:10

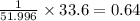 mol
mol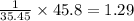 mol.
mol.
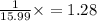 mol.
mol.
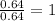
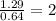
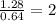
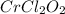
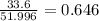
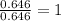 1
1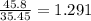
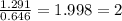 2
2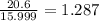
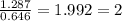 2
2

