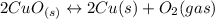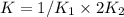Chemistry, 25.02.2020 06:14 andrewgainey1986
Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g) <--> 2Cu2O(s), K1 2CuO(s) <--> Cu2O(s) + 1/2 O2(g), K2 what is K for the system 2Cu(s) + O2(g) <--> 2CuO(s) equivalent to?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g) <--> 2Cu2O(s), K1...
Questions

Mathematics, 06.09.2021 09:00


Mathematics, 06.09.2021 09:00

Mathematics, 06.09.2021 09:00



Mathematics, 06.09.2021 09:00

World Languages, 06.09.2021 09:00



Advanced Placement (AP), 06.09.2021 09:10

Chemistry, 06.09.2021 09:10


Mathematics, 06.09.2021 09:10

Mathematics, 06.09.2021 09:10


Mathematics, 06.09.2021 09:10

Mathematics, 06.09.2021 09:10


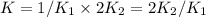
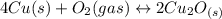
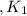 assuming equation (1)
assuming equation (1)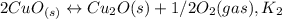 assuming equation (2)
assuming equation (2)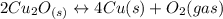 ,
,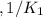 assuming equation (3)
assuming equation (3)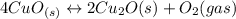
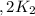 assuming equation (4)
assuming equation (4)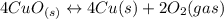
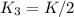 , equation (5)
, equation (5)