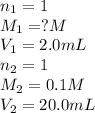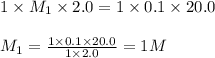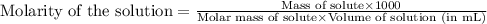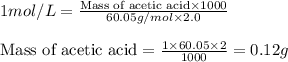Chemistry, 25.02.2020 05:12 rosetoheart2
You have a 2.0 mL sample of acetic acid (molar mass 60.05 g/mol) of unknown concentration. You titrate it to its endpoint with 20.0 mL NaOH (0.1 M). What mass of acetic acid was dissolved in the 2.0 mL of solution used?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 12:30
How does a nuclear reactor produce electricity? a. high-energy gamma rays are converted by a generator into electricity. b. the heat from the reaction turns water to steam, which runs a generator. c. the reaction produces a stream of electrons that flow through wires and into batteries. d. the heat released from the reaction is used to burn coal or gas to produce electricity. e. control rods absorb the neutrons emitted and release a stream of electrons as electricity.
Answers: 1

Chemistry, 23.06.2019 14:20
Timed ! in which of these statements are protons, electrons, and neutrons correctly compared? quarks are present in protons and neutrons but not in electrons. quarks are present in protons, neutrons, and electrons. quarks are present in neutrons and electrons but not in protons. quarks are present in protons and electrons but not in neutrons.
Answers: 1
You know the right answer?
You have a 2.0 mL sample of acetic acid (molar mass 60.05 g/mol) of unknown concentration. You titra...
Questions

English, 14.04.2021 05:30

History, 14.04.2021 05:30


Mathematics, 14.04.2021 05:30


Mathematics, 14.04.2021 05:30

Mathematics, 14.04.2021 05:30

History, 14.04.2021 05:30


English, 14.04.2021 05:30


Mathematics, 14.04.2021 05:30



Mathematics, 14.04.2021 05:30


Mathematics, 14.04.2021 05:30

Mathematics, 14.04.2021 05:30

Arts, 14.04.2021 05:30

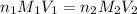
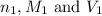 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 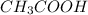
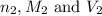 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.