
The proposed mechanism for a reaction is 1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW 2. I-(aq) + HClO(aq) => HIO(aq) + Cl-(aq) FAST 3. OH-(aq) + HIO(aq) => H2O(l) + IO-(aq) FAST Which of the following would be a rate law for the reaction? A. rate = k[ClO-][H2O] B. rate = k[I-][HClO] C. rate = k[OH-][HIO] D. rate = k[ClO-][H2O][I -] E. rate = k[ClO-][H2O][I-][OH-]

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1
You know the right answer?
The proposed mechanism for a reaction is 1. ClO-(aq) + H2O(l) => HClO(aq) + OH-(aq) SLOW 2. I-(aq...
Questions




Mathematics, 03.10.2019 00:00


Social Studies, 03.10.2019 00:00


Mathematics, 03.10.2019 00:00

History, 03.10.2019 00:00

Physics, 03.10.2019 00:00

Physics, 03.10.2019 00:00


Mathematics, 03.10.2019 00:00

Mathematics, 03.10.2019 00:00

Mathematics, 03.10.2019 00:00

English, 03.10.2019 00:00




Mathematics, 03.10.2019 00:00

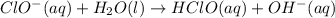
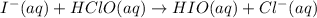
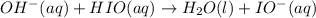
![R=k[ClO^-][H_2O]](/tpl/images/0522/8081/8de77.png)


