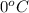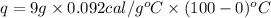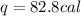Chemistry, 25.02.2020 04:30 hela9astrid
The specific heat capacity of copper is 0.092 calories per gram per degree Celsius. How much heat is required to raise the temperature of a 9 g piece of copper from 0°C to 100°C?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
The specific heat capacity of copper is 0.092 calories per gram per degree Celsius. How much heat is...
Questions


Biology, 26.01.2021 21:20

English, 26.01.2021 21:20


Mathematics, 26.01.2021 21:20

History, 26.01.2021 21:20

Mathematics, 26.01.2021 21:20



Mathematics, 26.01.2021 21:20

History, 26.01.2021 21:20

English, 26.01.2021 21:20




History, 26.01.2021 21:20


Mathematics, 26.01.2021 21:20


Mathematics, 26.01.2021 21:20

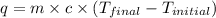
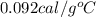
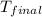 = final temperature =
= final temperature = 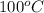
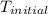 = initial temperature =
= initial temperature =