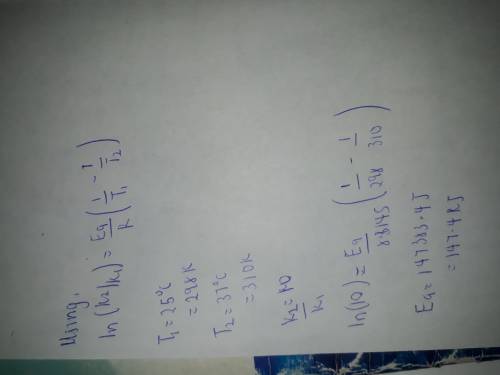Chemistry, 25.02.2020 04:33 floressavanna15
If a temperature increase from 25.0 0 O 2 ` (g) → H 2 C to 37.0 0 O(l) + O 2 (g) C increases the rate constant of a reaction by ten-fold, what is the value for the activation energy of the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1


Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
If a temperature increase from 25.0 0 O 2 ` (g) → H 2 C to 37.0 0 O(l) + O 2 (g) C increases the rat...
Questions


History, 03.12.2019 21:31

Social Studies, 03.12.2019 21:31


History, 03.12.2019 21:31






English, 03.12.2019 21:31


Mathematics, 03.12.2019 21:31



Mathematics, 03.12.2019 21:31


Chemistry, 03.12.2019 21:31

Biology, 03.12.2019 21:31