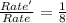Chemistry, 25.02.2020 04:05 alialbinn6969
The gas phase reaction between NO and O2 is second order with respect to NO and first order with respect to 02, what would happen to the reaction rate if the concentrations of both reactants were halved with everything else held constant?
a. It would decrease by a factor of 8 It would remain unchanged.
b. It would decrease by a factor of 16.
c. It would decrease by a factor of 4.
d. It would decrease by a factor of 2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

You know the right answer?
The gas phase reaction between NO and O2 is second order with respect to NO and first order with res...
Questions



History, 03.08.2020 14:01

SAT, 03.08.2020 14:01






Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

Advanced Placement (AP), 03.08.2020 14:01




Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01

![Rate=k[NO]^2[O_2]^1](/tpl/images/0522/7517/1df0d.png) (1)
(1)![Rate'=k{\frac{[NO]}{2}^2}{\frac{[O_2]}{2}^1}](/tpl/images/0522/7517/19c10.png) (2)
(2)![\frac{Rate'}{Rate}=\frac{k\frac{[NO]}{2}^2\times \frac{([O_2]}{2})^1}{k[NO]^2[O_2]^1}](/tpl/images/0522/7517/9c613.png)