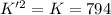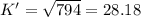Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
The value of for the equilibrium H2 (g) + I2(g) 2 HI (g) is 794 at 25 °C. What is the value of for t...
Questions




Mathematics, 24.02.2021 06:40



Mathematics, 24.02.2021 06:40

History, 24.02.2021 06:40

Mathematics, 24.02.2021 06:40


Biology, 24.02.2021 06:40



History, 24.02.2021 06:40

Mathematics, 24.02.2021 06:40

Mathematics, 24.02.2021 06:40


Social Studies, 24.02.2021 06:40


Mathematics, 24.02.2021 06:40

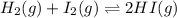
![K=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0522/6838/8a740.png)
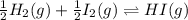
![K'=\frac{[HI]}{[H_2]^{\frac{1}{2}}[I_2]^{\frac{1}{2}}}](/tpl/images/0522/6838/5f7dd.png)
![K'^2=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0522/6838/999fb.png)