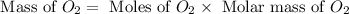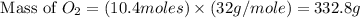Chemistry, 25.02.2020 03:49 springlover7266
The combustion of propane may be described by the chemical equation C 3 H 8 ( g ) + 5 O 2 ( g ) ⟶ 3 CO 2 ( g ) + 4 H 2 O ( g ) How many grams of O 2 ( g ) are needed to completely burn 91.6 g C 3 H 8 ( g ) ?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
The combustion of propane may be described by the chemical equation C 3 H 8 ( g ) + 5 O 2 ( g ) ⟶ 3...
Questions




Mathematics, 03.09.2021 14:20

Business, 03.09.2021 14:20

Mathematics, 03.09.2021 14:20


Mathematics, 03.09.2021 14:30



English, 03.09.2021 14:30

Mathematics, 03.09.2021 14:30


Business, 03.09.2021 14:30

Geography, 03.09.2021 14:30



Physics, 03.09.2021 14:30

World Languages, 03.09.2021 14:30

Social Studies, 03.09.2021 14:30

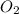 needed are, 332.8 grams
needed are, 332.8 grams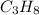 = 91.6 g
= 91.6 g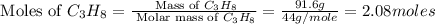
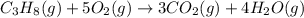
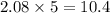 moles of
moles of