
Chemistry, 25.02.2020 03:42 delaneynagle3368
Which of the following compounds has dipole-dipole interactions as the strongest intermolecular force? Which of the following compounds has dipole-dipole interactions as the strongest intermolecular force? CBr4 BrCH2CH2OH CH3Br Br2

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

You know the right answer?
Which of the following compounds has dipole-dipole interactions as the strongest intermolecular forc...
Questions


Mathematics, 06.10.2019 15:30

Mathematics, 06.10.2019 15:30

English, 06.10.2019 15:30


Social Studies, 06.10.2019 15:30

Chemistry, 06.10.2019 15:30



History, 06.10.2019 15:30


Geography, 06.10.2019 15:30


Mathematics, 06.10.2019 15:30




History, 06.10.2019 15:30

Mathematics, 06.10.2019 15:30

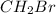
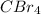 , electronegativities of carbon (C) and bromine (Br) are different and four dipoles exist in the molecules but the net vector sum of all the dipoles are zero. Therefore, its is a non-polar molecule.
, electronegativities of carbon (C) and bromine (Br) are different and four dipoles exist in the molecules but the net vector sum of all the dipoles are zero. Therefore, its is a non-polar molecule.
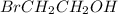 , the strongest intermolecular force is hydrogen bonding.
, the strongest intermolecular force is hydrogen bonding.
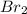 , same atoms are bonded, therefore, it is a non-polar molecule and hence, cannot has dipole-dipole interaction.
, same atoms are bonded, therefore, it is a non-polar molecule and hence, cannot has dipole-dipole interaction.


