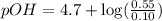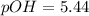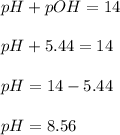Chemistry, 25.02.2020 02:57 fangirl2837
What is the pH of a solution that results when 0.010mol HNO3 is added to 500.ml of a solution that is 0.10M in aqueous ammonia and 0.55 M in ammonium nitrate. assume no volume change. (The Kb for NH3 =1.8 * 10-5 )

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
You know the right answer?
What is the pH of a solution that results when 0.010mol HNO3 is added to 500.ml of a solution that i...
Questions


Mathematics, 19.03.2021 15:50





Mathematics, 19.03.2021 15:50

English, 19.03.2021 15:50



English, 19.03.2021 16:00

Mathematics, 19.03.2021 16:00



Computers and Technology, 19.03.2021 16:00


Mathematics, 19.03.2021 16:00



Mathematics, 19.03.2021 16:00

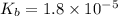
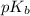 .
.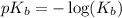
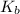 in this expression, we get:
in this expression, we get: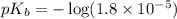
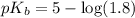
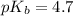
![pOH=pK_b+\log \frac{[Salt]}{[Base]}](/tpl/images/0522/5805/ac570.png)