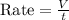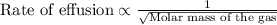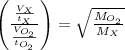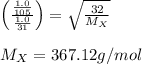A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. It required 105 s for 1.0 L of the gas to effuse. Under identical experimental conditions it required 31 s for 1.0 L of O2 gas to effuse. Calculate the molar mass of the unknown gas.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressur...
Questions

Mathematics, 21.09.2019 23:50


Mathematics, 21.09.2019 23:50

Biology, 21.09.2019 23:50



Mathematics, 21.09.2019 23:50



Social Studies, 21.09.2019 23:50



Biology, 21.09.2019 23:50


Biology, 21.09.2019 23:50

Mathematics, 21.09.2019 23:50

Biology, 21.09.2019 23:50

Biology, 21.09.2019 23:50