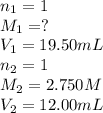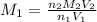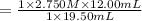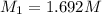Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
A student receives a solution of 2.750 M NaOH to titrate with 12.00 mL of an unknown solution of HCl...
Questions


Mathematics, 14.07.2019 02:00

Mathematics, 14.07.2019 02:00

Mathematics, 14.07.2019 02:00

Mathematics, 14.07.2019 02:00

Mathematics, 14.07.2019 02:00

Mathematics, 14.07.2019 02:00

Geography, 14.07.2019 02:00



History, 14.07.2019 02:00



History, 14.07.2019 02:00

Biology, 14.07.2019 02:00



Mathematics, 14.07.2019 02:00

Social Studies, 14.07.2019 02:00


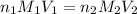
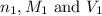 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 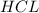
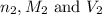 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.