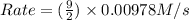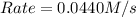The reaction A + B ⟶ C + D rate = k [ A ] [ B ] 2 has an initial rate of 0.0440 M / s. What will the initial rate be if [ A ] is halved and [ B ] is tripled? initial rate: .00978 M / s What will the initial rate be if [ A ] is tripled and [ B ] is halved? initial rate:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
The reaction A + B ⟶ C + D rate = k [ A ] [ B ] 2 has an initial rate of 0.0440 M / s. What will the...
Questions



Mathematics, 07.04.2020 04:56



History, 07.04.2020 04:56


Mathematics, 07.04.2020 04:56



Mathematics, 07.04.2020 04:56

English, 07.04.2020 04:56


Geography, 07.04.2020 04:56



Mathematics, 07.04.2020 04:56


Mathematics, 07.04.2020 04:56

![Rate=k[A][B]^2](/tpl/images/0522/4296/d8f90.png)
![Rate=k\times (\frac{[A]}{2})\times (3\times [B])^2](/tpl/images/0522/4296/31c7e.png)
![Rate=k\times (\frac{[A]}{2})\times 9\times [B]^2](/tpl/images/0522/4296/e768d.png)
![Rate=k\times (\frac{9}{2})\times [A]\times [B]^2](/tpl/images/0522/4296/4e890.png)
![k[A][B]^2](/tpl/images/0522/4296/8ef61.png) = 0.0440 M/s
= 0.0440 M/s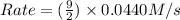
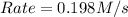
![Rate=k\times (\frac{[B]}{2})\times (3\times [A])^2](/tpl/images/0522/4296/e26df.png)
![Rate=k\times (\frac{[B]}{2})\times 9\times [A]^2](/tpl/images/0522/4296/e83c0.png)