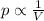Chemistry, 25.02.2020 00:10 stephb1369
In response to Boyle’s Law, the pressure of a gas increases as the volume decreases because
A. the gas particles strike the walls of the container more often
B. the temperature of the gas increases
C. the gas particles get bigger
D. the gas particles strike the walls of the container with more force

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3


Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1
You know the right answer?
In response to Boyle’s Law, the pressure of a gas increases as the volume decreases because
Questions



Chemistry, 23.07.2019 21:30


Chemistry, 23.07.2019 21:30

History, 23.07.2019 21:30

Chemistry, 23.07.2019 21:30


History, 23.07.2019 21:30

History, 23.07.2019 21:30

History, 23.07.2019 21:30


History, 23.07.2019 21:30

Social Studies, 23.07.2019 21:30

Social Studies, 23.07.2019 21:30

English, 23.07.2019 21:30

Mathematics, 23.07.2019 21:30


Geography, 23.07.2019 21:30

History, 23.07.2019 21:30