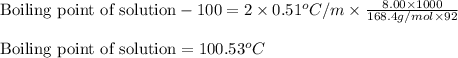Chemistry, 24.02.2020 23:27 ashleybarrera2000
An aqueous CsCl solution is 8.00 wt% CsCl and has a density of 1.0643 g/mL at 20°C. What is the boiling point of this solution? Kb = 0.51°C/m for water. Enter your answer with 2 decimal places and no units.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 12:30
Choose one literary selection from this semester in which you think the setting has a great impact on the work. in a full paragraph name the work, describe the setting, and explain why it is so important to the overall story or poem.
Answers: 1

Chemistry, 23.06.2019 21:00
Which is greater in positive acceleration, initial or final velocity
Answers: 1

Chemistry, 23.06.2019 21:50
Agas engine that operates on a brayton cycle has an efficiency of 0.23. on a cold day, the temperature of the air drawn into the engine is 267 k.part awhat is the temperature of the air exhausted from the engine?
Answers: 3
You know the right answer?
An aqueous CsCl solution is 8.00 wt% CsCl and has a density of 1.0643 g/mL at 20°C. What is the boil...
Questions


English, 06.05.2020 05:35



Mathematics, 06.05.2020 05:35

History, 06.05.2020 05:35


History, 06.05.2020 05:35


History, 06.05.2020 05:35




Mathematics, 06.05.2020 05:35



Mathematics, 06.05.2020 05:35



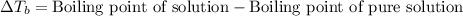
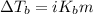
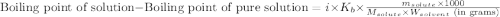
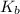 = molal boiling point elevation constant = 0.51°C/m
= molal boiling point elevation constant = 0.51°C/m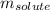 = Given mass of solute (CsCl) = 8.00 g
= Given mass of solute (CsCl) = 8.00 g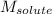 = Molar mass of solute (CsCl) = 168.4 g/mol
= Molar mass of solute (CsCl) = 168.4 g/mol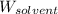 = Mass of solvent (water) = 92 g
= Mass of solvent (water) = 92 g