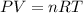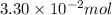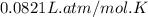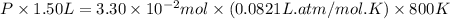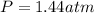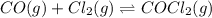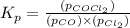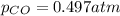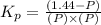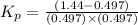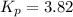Chemistry, 24.02.2020 22:28 pattydixon6
Pure phosgene gas (COCl2,), 3.30 x 10-2 mol, was placed in a 1.50-L container. It was heated to 800K, and at equilibrium the pressure of CO was found to be 0.497 atm. Calculate the equilibrium constant, Kp, for the reaction: CO(g) Cl2(g) COCl2(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1

Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1
You know the right answer?
Pure phosgene gas (COCl2,), 3.30 x 10-2 mol, was placed in a 1.50-L container. It was heated to 800K...
Questions

Mathematics, 27.01.2020 06:31

Mathematics, 27.01.2020 06:31

Physics, 27.01.2020 06:31

History, 27.01.2020 06:31


Physics, 27.01.2020 06:31

Biology, 27.01.2020 06:31

History, 27.01.2020 06:31




Chemistry, 27.01.2020 06:31

Mathematics, 27.01.2020 06:31


English, 27.01.2020 06:31

Mathematics, 27.01.2020 06:31


Mathematics, 27.01.2020 06:31


History, 27.01.2020 06:31

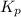 for the reaction is, 3.82
for the reaction is, 3.82